
 Blending process monitoring with in-line NIR spectroscopy
Blending process monitoring with in-line NIR spectroscopy
 RESCHAPE PROJECT: RESHAPING SUPPLY CHAINS FOR POSITIVE SOCIAL IMPACT
RESCHAPE PROJECT: RESHAPING SUPPLY CHAINS FOR POSITIVE SOCIAL IMPACT

Controllo del processo di rivestimento di forme granulari mediante spettroscopia NIR

Controllo del processo di rivestimento di forme granulari mediante spettroscopia NIR
Nell’industria farmaceutica esistono molte formulazioni granulari che vengono rivestite per ottenere un rilascio prolungato o controllato del farmaco o dell’ingrediente farmaceutico attivo (API) nel tempo; un esempio chiaro e noto è l’omeprazolo. In questo articolo discuteremo di queste formulazioni a rilascio prolungato e di come sia possibile ottimizzare il tempo di rilascio e le analisi di potenza durante il processo di rivestimento utilizzando la spettroscopia NIR.

Processo di pellettizzazione e analisi tradizionale
Durante il processo di pellettizzazione delle forme di dosaggio a rilascio modificato, la corretta applicazione del rivestimento (ad esempio un rivestimento a rilascio enterico destinato a prevenire la digestione o la degradazione gastrica) determinerà la successiva efficacia del farmaco e il tempo di rilascio mg/API del farmaco; pertanto, durante tutto il processo vengono effettuati controlli per garantire la qualità e quindi l’azione farmacologica attesa.
Attualmente, questo controllo viene effettuato durante il processo di rivestimento con campioni ottenuti dall’apparecchiatura di rivestimento in tempi diversi e analizzati in laboratorio utilizzando la tecnica analitica dell’HPLC o della cromatografia liquida e il test di dissoluzione per dimostrare che il rilascio del/i principio/i attivo/i è soddisfacente. Entrambi i metodi richiedono la preparazione del campione prima dell’analisi, richiedono personale specializzato e materiali di consumo (materiali), oltre alla durata (ore) di un test di dissoluzione, il cui obiettivo principale è quello di determinare la biodisponibilità del farmaco, ovvero la quantità relativa di farmaco che è entrata nella circolazione generale dopo la somministrazione e la velocità con cui questo accesso è avvenuto.
Pertanto, il problema principale dell’analitica tradizionale è che richiede molto tempo per ottenere i risultati e quindi non consente di correggere tempestivamente il processo di rivestimento in caso di insuccessi o, nel caso frequente di interruzione del processo per il campionamento, c’è il rischio di alterare la qualità del semiprodotto.
Uno strumento alternativo e molto efficace che consente di monitorare in tempo reale il processo di rivestimento è la tecnologia NIR, in quanto la firma spettrale di ciascun pellet può essere messa in relazione con le condizioni di rivestimento, il dosaggio e i tempi di rilascio senza dover ricorrere a metodi tradizionali.
Sviluppo di un metodo NIRS per la previsione del tempo di rilascio e della potenza
Per sviluppare un modello predittivo per la determinazione in tempo reale dei tempi di rilascio e della potenza (mg API/g pellet) rilasciata a 1, 4 e 7 ore, abbiamo lavorato in coordinamento con un importante laboratorio farmaceutico spagnolo e con l’analizzatore spettroscopico NIR portatile Visum Palm™ prodotto e commercializzato da IRIS Technology Solutions S.L.
I dati forniti dal laboratorio consistono negli spettri NIR di diversi lotti di due farmaci a base, da un lato, di un antistaminico che, per motivi di riservatezza, chiameremo “DS” e, dall’altro, di una forma di vitamina B6 che, per gli stessi motivi, chiameremo “PH”. In entrambi i casi, il principio attivo faceva parte del rivestimento dei pellet che costituiscono il veicolo.
Gli spettri dei pellet sono stati acquisiti in diversi momenti del processo di rivestimento, sia da campioni umidi che da campioni secchi e, parallelamente, il rispettivo campione è stato sottoposto alle analisi consuete in questi casi per determinare il rilascio del farmaco a 1, 4 e 7 ore e la potenza mg PI/g.
I modelli predittivi sviluppati sulla base dei dati spettrali hanno dimostrato che non è necessario asciugare i campioni per l’acquisizione degli spettri – quindi il controllo può essere eseguito direttamente sul campione umido, risparmiando tempo e manipolazione – e che esiste una chiara relazione tra gli spettri NIR, la potenza e i tempi di rilascio di 1h, 4h e 7h, come vedremo di seguito.
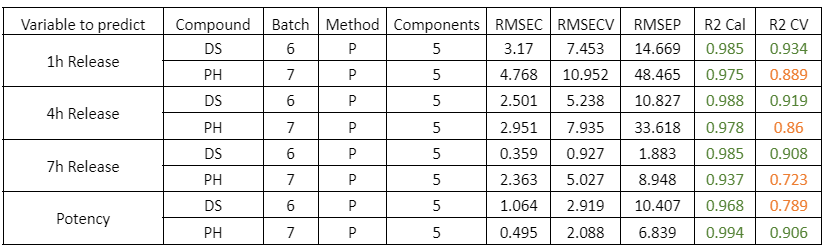
Composto PH
Tabella 1: Parametri qualitativi dei modelli di previsione per il rilascio a 1, 4, 7 ore e la potenza nei campioni con diverse fasi del processo di rivestimento PH. Il simbolo * indica che il modello è stato costruito utilizzando gli spettri NIR medi delle repliche di ciascun campione.
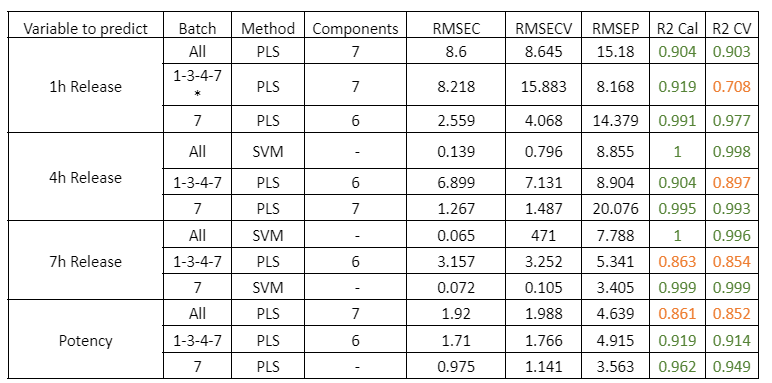
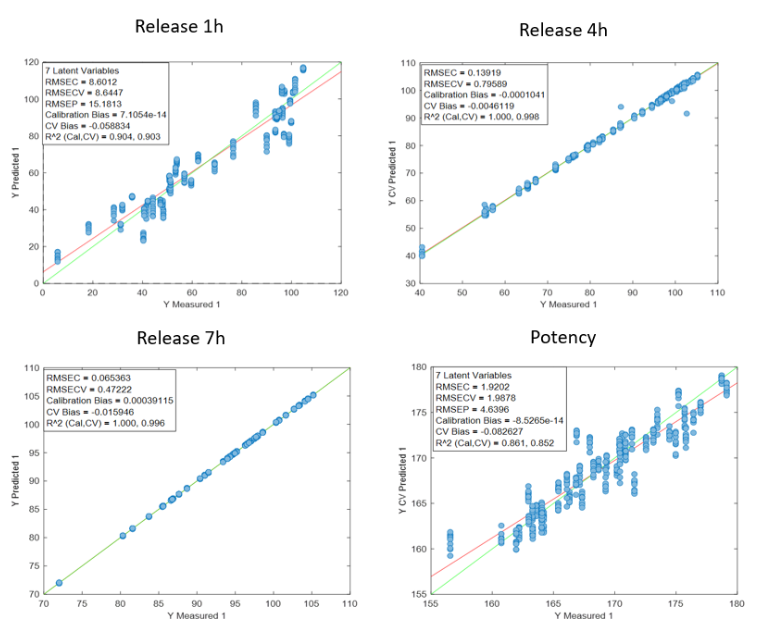
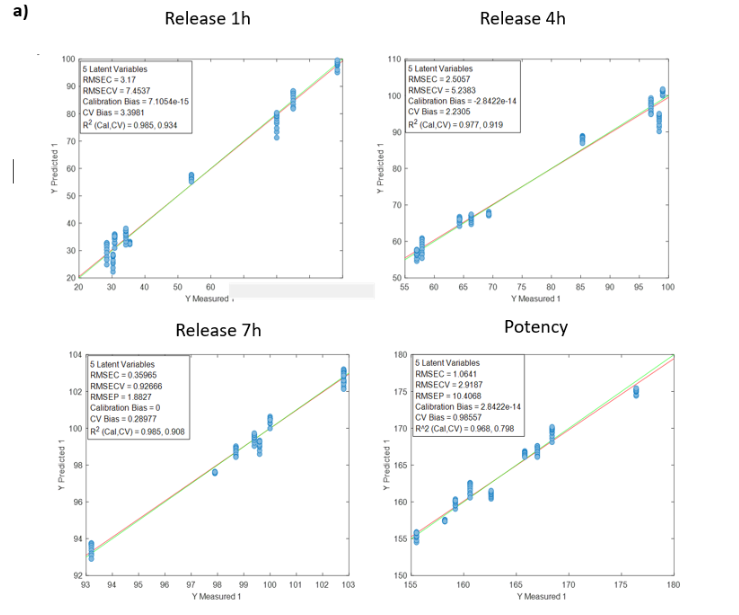
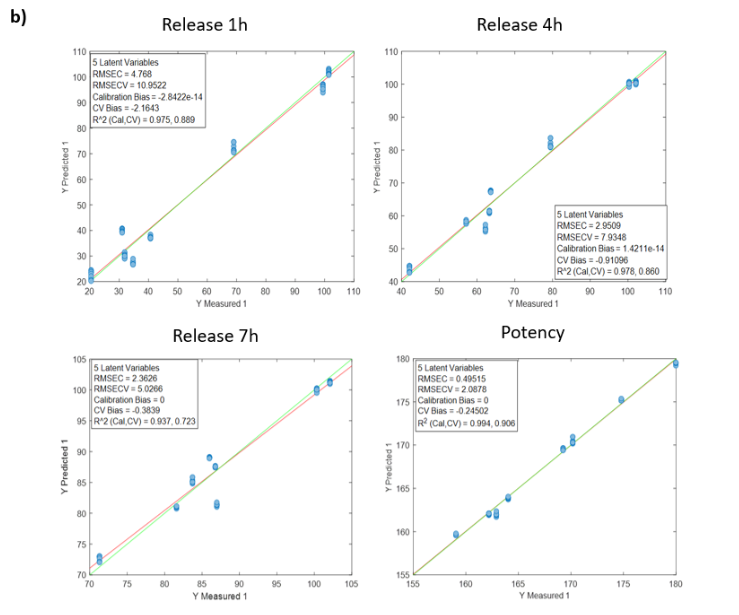
Figura 1: Curve di regressione per PH a) Tutti i campioni; b) Lotti 1,3,4 y 7; c) Spettri medi dei lotti 1,3,4 y 7; d) Lotto 7.
Composto DS
La Tabella 2 mostra i parametri di qualità dei modelli per l’analisi dei campioni di DS umido. Tutti i campioni sono stati studiati contemporaneamente: i campioni dei lotti 6, 8 e 10 insieme e il lotto 6 separatamente. I lotti 6, 8 e 10 sono stati scelti per lo studio di un insieme di lotti perché presentavano il maggior numero di campioni. Inoltre, il lotto 6 è stato scelto per l’analisi individuale in quanto conteneva il maggior numero di campioni con i parametri di rilascio ottimali per il caso di studio.
Tabella 2: Parametri qualitativi dei modelli di previsione per il rilascio a 1, 4, 7 ore e la potenza nei campioni con diverse fasi del processo di rivestimento DS.
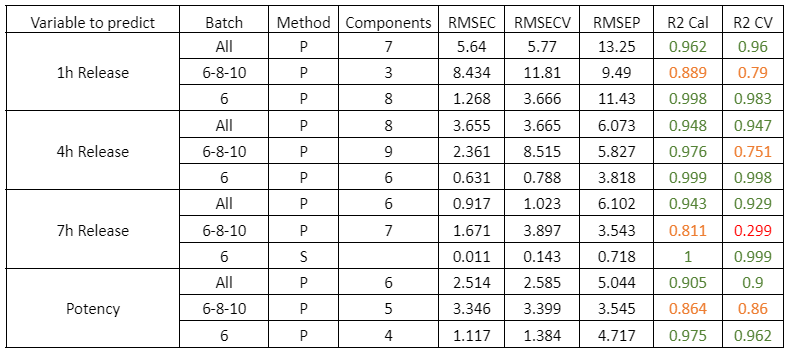
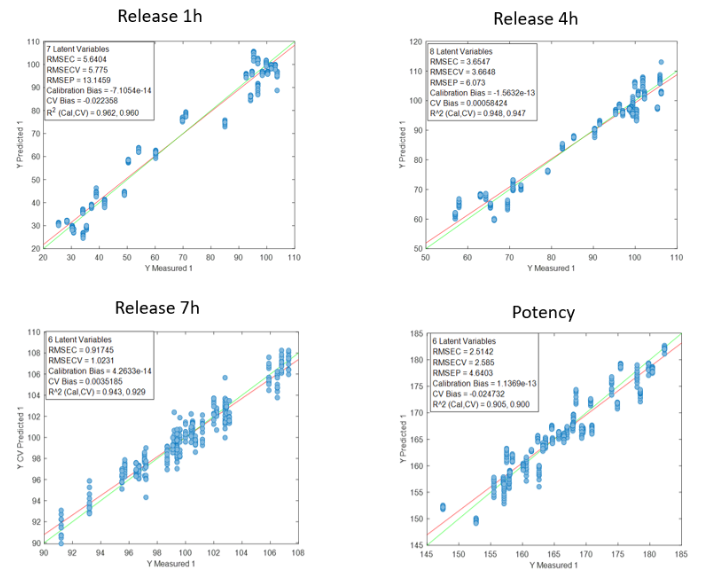
La Figura 2 mostra le curve di regressione risultanti dallo studio per il principio attivo DS. I valori dei parametri di qualità per i modelli DS mostrano, in generale, una buona correlazione. Come osservazione, si nota che l’errore aumenta quando si utilizzano dati di lotti diversi, probabilmente perché le condizioni di processo di ciascun lotto sono diverse a causa del fatto che i dati provengono dalla fase di sviluppo e messa a punto del processo produttivo. La previsione del rilascio a 7 ore è peggiore di quella degli altri parametri, probabilmente perché in molti casi la fine del processo di rilascio è stata raggiunta prima di quel momento.
Figura 2: Curve di regressione per DS a) tutti i campioni; b) Lotti 6, 8 y 10; c) Spettri medi dei lotti 6, 8 y 10; d) Lotto 6.
Previsione di campioni secchi
Tabella 3: Parametri qualitativi dei modelli di previsione per i campioni secchi di DS lotto 6 e PH lotto 7.
I modelli di previsione dei campioni secchi per i singoli lotti di PH e DS mostrano una buona correlazione. Va notato che l’errore di previsione è dovuto ai pochi campioni di convalida utilizzati.
Figura 3: Curve di regressione per i campioni secchi di a) DS lotto 6 y b) PH lotto 7.
Conclusioni
- Esiste una chiara correlazione tra gli spettri NIR e i tempi di rilascio di 1h, 4h e 7h, nonché con la potenza, sia per DS che per PH, sebbene sia leggermente peggiore per PH.
- Nel caso del rilascio a 7 ore, la correlazione sembra un po’ più debole, forse perché è vicino al rilascio massimo (al plateau di rilascio) o a causa delle differenze nel pH dei campioni.
- Le diverse condizioni di produzione dei lotti influenzano la robustezza di questa correlazione, un fattore di variabilità intrinseco perché i campioni provengono dalla fase di sviluppo del processo produttivo (fase di messa a punto) e non dal metodo NIRS.
- I test dei singoli lotti mostrano una buona correlazione sia per i campioni umidi che per quelli secchi. Poiché i risultati in entrambi i casi sono simili, si può concludere che l’essiccazione non è necessaria per correlare i parametri studiati (tempo di rilascio e potenza) con gli spettri NIR.
- Infine, dall’analisi dei risultati analizzati, si può concludere che la spettroscopia NIR può essere utilizzata per ottimizzare il controllo del processo di rivestimento delle forme granulari e che, da un punto di vista tecnico, si tratta di un metodo robusto e basato sull’evidenza. Tuttavia, per tutti i casi valutati in questo documento, i modelli definitivi devono essere realizzati una volta che il processo di produzione è stato completamente sviluppato.



