
 Raman Spectroscopy in Liposome Diafiltration Process
Raman Spectroscopy in Liposome Diafiltration Process
 IRIS Technology: Leading the Way in Material Detection with Hyperspectral Imaging for RECLAIM project
IRIS Technology: Leading the Way in Material Detection with Hyperspectral Imaging for RECLAIM project

Pharmaceutical Raw Material Identification (RMID) with Visum Palm GxP™

Pharmaceutical Raw Material Identification (RMID) with Visum Palm GxP™
Raw material identification or verification analysis is a critical process in the pharmaceutical industry, as it ensures the identity and quality of all materials that will be used in production. A practical and efficient way to perform it is through the Visum Palm GxP™ portable NIR analyzer, which allows the test to be carried out directly in the warehouse and provides accurate results in less than 3 seconds, avoiding laboratory overload and reducing production cycle times.
There are multiple technologies commonly used for raw material identification (NIR, FT-NIR or Raman), but none of them alone constitutes a universal solution that covers the entire list of materials of a pharmaceutical manufacturer.
NIR technology is especially effective in the identification of organic compounds, although it can also be applied to certain inorganic materials under very specific conditions, such as in the case of hygroscopic substances (salts), hydrated compounds or those dissolved in aqueous solution.
On the other hand, Raman spectroscopy represents an alternative technique with its own limitations, especially in:
• Organic compounds with fluorescence (colorants and certain APIs).
• Substances with weak Raman signals.
• Purely ionic inorganic compounds (NaCl, KCl, HCl).
In general, NIR is widely established in regulated environments due to its effectiveness, immediacy and ease of use by any operator. In addition, it does not require additional safety measures (as in the case of Raman, where protective glasses are needed) and is a much more economical option for pharmaceutical raw material identification and quantitative analysis.



Why Visum Palm GxP™ for pharmaceutical raw material identification?
Visum Palm GxP is a self-contained analyzer that does not need to connect or link to any external device (smartphone, computer, tablet or other).
It has dual functionality, allowing its use both as a handheld portable device (in direct contact with the sample) and in benchtop mode. It offers a spectral resolution of 5 nm or 256 pixels, placing it as the portable NIR analyzer with the highest spectral capacity in its category.
It complies with the main international regulatory frameworks: CFR 21 Part 11, USP 1119, Ph. Eur. 2.2.40, JP18 and GAMP 5. As a standalone system with class 4 software, the pharmaceutical qualification procedure is much more agile and simple compared to other analyzers that depend on external software or additional processing units.
The measurement area is 10 mm in diameter, with an illumination area of 50 mm, which ensures greater chemical information and makes it an especially useful solution for raw material identification in large and translucent bags.
The system includes a set of accessories that allow working in benchtop mode or as a handheld analyzer, including adapters for laboratory spectrophotometry cuvettes and vials for liquids and powders. In addition, it has an optical reducer designed for tablet inspection and other reduced analysis areas.
Visum Palm GxP is not only optimized for pharmaceutical raw material identification, but also offers support for quantitative analysis. Automatically generated technical reports follow the guidelines of ICH Q2(R2) – Validation of Analytical Procedures – Scientific Guideline, ensuring traceability and regulatory compliance.
Figure 1: Support base for working in benchtop mode with Visum Palm GxP™. Allows coupling of benchtop sample holders for powders, solids, granules and liquids.
GxP and CFR 21 Part 11 functionalities
Visum Palm GxP integrates the most complete and up-to-date set of functionalities specifically designed to guarantee raw material identification and regulatory compliance in pharmaceutical environments regulated by GMP. Its software and hardware architecture positions it as the reference tool to ensure quality, traceability and analytical reliability at every stage of the process.
Among its main features:
• Complete Audit Trail: detailed recording of both the device and the results, with encrypted binary files for maximum security.
• Advanced role and privilege management: three predefined levels (Analyst, Supervisor and User Manager) with the possibility of creating unlimited users.
• Secure access control: robust passwords with intuitive modification and restoration procedures.
• Configurable time parameters: session time and password expiration management.
• Flexible time synchronization: multiple methods to ensure accurate date and time on the device.
• Integrated qualification assistant: guided validation of wavelength accuracy, noise and photometric linearity.
• Dual-level electronic signature: with independent validation by Analyst and Supervisor.
• Backup and restoration procedures: designed to maintain system integrity and operational continuity.
Thanks to this set of functionalities, Visum Palm GxP is consolidated as the most robust portable analyzer on the market, ensuring not only compliance with international regulations (CFR 21 Part 11, GAMP 5, pharmacopeias), but also maximum efficiency in pharmaceutical raw material identification and quantitative analysis applications.
Analysis modes with Visum Palm GxP™ for raw material identification
The Visum Palm GxP™ analyzer enables raw material identification or verification analysis in less than 3 seconds for different compounds in solid, granular, powder or liquid state. The procedure consists of comparing the spectrum acquired from the sample with the reference spectra stored in the library.
The comparison is carried out through a mathematical similarity criterion (HQI, Hit Quality Index), which converts spectral differences into a numerical value. As a result, the device provides the substance or class with the highest degree of similarity obtained (Figure 3) and displays a list of the other substances ordered from highest to lowest match.
Likewise, the system allows a verification analysis, previously selecting from the library the specific material whose identity is to be confirmed. In this case, the result is presented as PASS or FAIL and, in case of FAIL, the analyzer also indicates the correct substance with the highest similarity (Figure 4).
All results, including discarded ones or those that have not been electronically signed by the user using credentials, are stored in the encrypted Audit Trail, ensuring traceability, data integrity and GMP regulatory compliance.
Main screens of Visum Palm™
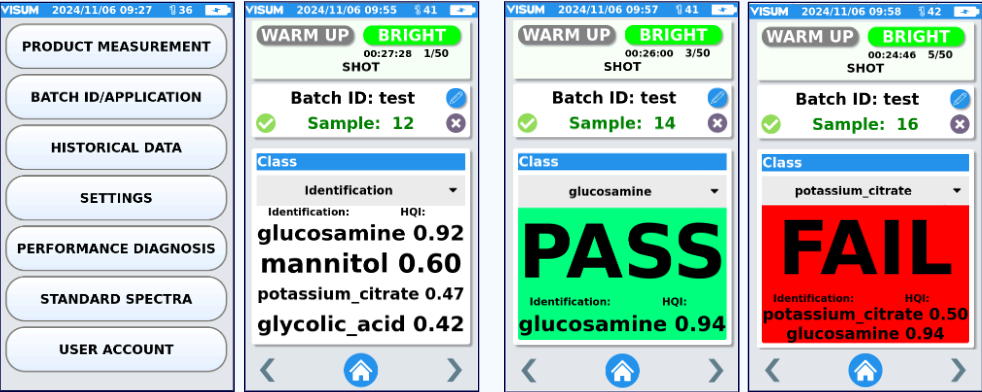
Figure 2: Main Menu Figure 3: Raw material identification analysis Figure 4: Verification analysis, PASS and FAIL with confirmation of correct substance.
In specific cases it may occur that the technology does not provide an exact result, as happens when inspecting chemically very similar or practically identical materials, or when there are differences in granulometry. For these situations, Visum Palm GxP™ offers the possibility of creating and using classification libraries.
Unlike identification/verification modes, classification (Figure 5) allows precise distinction of very subtle spectral differences, such as particle size or the concentration of a specific analyte, even when dealing with the same API or excipient present in different concentrations within similar matrices. In this way, classification complements the initial raw material identification analysis through a more powerful algorithm for complex or problematic cases.
In all the scenarios described, in addition to the result, the system generates the complete spectrum of the analyzed substance in each measurement (Figure 6), ensuring traceability and greater analytical value.
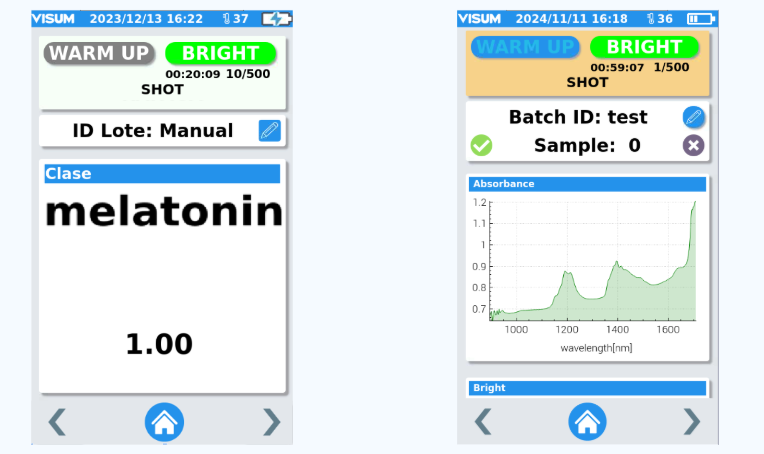
Figure 5: Classification analysis. Figure 6: Spectrum of each analysis.
Develop raw material identification methods and libraries with Visum Master GMP™ software for PC
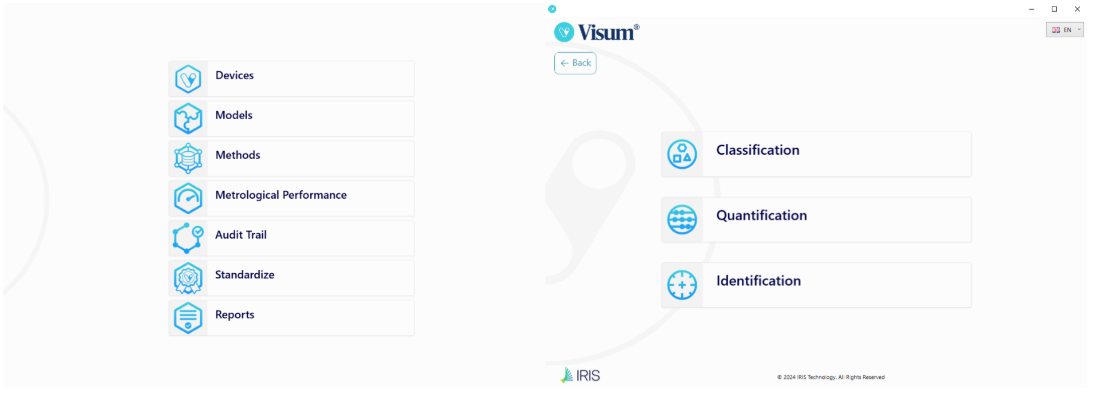
Figure 7: Visum Master™ – Main Menu Figure 8: Visum Master™ – Model Builder
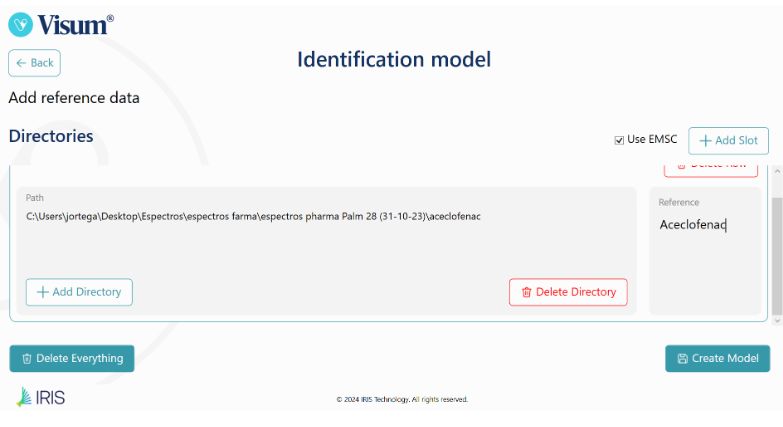
Figure 9: Insert the spectra of each material and the reference (name) to create the raw material identification library.
Visum Palm GxP™ is the only NIR analyzer on the market that allows the end user to easily and fully automatically develop their own raw material identification libraries, classification libraries or even methods for quantitative tests, without the need to use complex software or have prior knowledge in chemometrics. In other words, the user only needs to have samples and qualitative or quantitative references, leaving the integrated Model Builder to handle the development process.
Generating, maintaining or updating libraries has never been as simple as with Visum Master™, since it allows generating unlimited libraries and methods for different types of analysis, and for each one it also automatically generates a technical report with all the information on how it was done, the algorithm used, pretreatments, latent variables, quality metrics, dataset used, outlier quality control, graph and quantification of detected and eliminated outliers, among other data that are a key input to prepare an internal or external validation report, identify improvement needs or updates.
To learn more about how Visum Palm GxP can be a useful tool for raw material identification, you can contact IRIS or our distributors through the following link.




