RESCHAPE PROJECT: RESHAPING SUPPLY CHAINS FOR POSITIVE SOCIAL IMPACT
RESCHAPE PROJECT: RESHAPING SUPPLY CHAINS FOR POSITIVE SOCIAL IMPACT
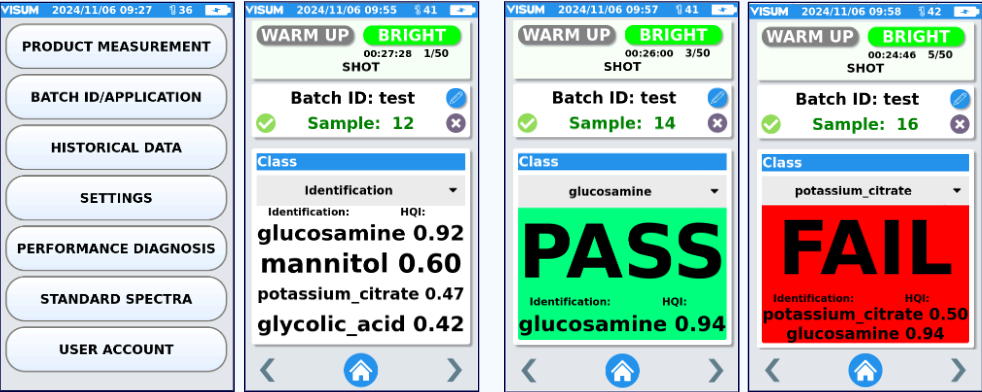
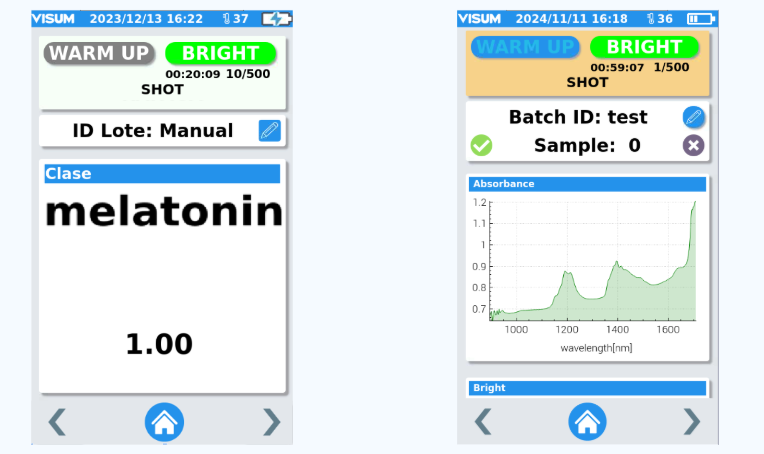
 Pharmaceutical Raw Material Analysis (RMID) with Visum Palm GxP™
Pharmaceutical Raw Material Analysis (RMID) with Visum Palm GxP™

Raman Spectroscopy in Liposome Diafiltration Process

Raman Spectroscopy in Liposome Diafiltration Process
The pharmaceutical industry frequently relies on the encapsulation of active pharmaceutical ingredients (APIs) in liposomal systems to improve their stability, bioavailability, and controlled release. A key step in this process is diafiltration, which removes unwanted compounds after liposome formation, including organic solvents like ethanol, which is used to dissolve the lipids that form the liposomal bilayer. Accurate quantification of residual ethanol using Raman spectroscopy-based analyzers is essential to ensure the final product quality, meet regulatory standards, and optimize process time and resources.
Raman Spectroscopy and the Visum Raman In-Line™ System
Raman spectroscopy is a vibrational technique based on the inelastic scattering of laser light. It is highly specific for each molecule, making it ideal for the identification and quantification of chemical components, even in complex matrices. The Visum Raman In-Line™ system, developed by IRIS Technology Solutions, allows the application of Raman spectroscopy directly in industrial or laboratory settings thanks to its compact, robust, and adaptable design. It eliminates the need for sample preparation, enables fast, real-time analysis, and is ideal for continuous monitoring of pharmaceutical processes with high regulatory demands.
Quantification of Residual Ethanol by Raman Spectroscopy
This project focused on implementing a residual ethanol quantification model using Raman spectroscopy during the diafiltration process following liposome formation. For confidentiality reasons, the two encapsulated active ingredients analyzed are referred to as 1: NSAID (non-steroidal anti-inflammatory drug) and 2: Biomolecule. Samples were analyzed under controlled laboratory conditions using the Visum Raman In-Line™ system. The Raman system was mounted on a mobile rack, with a sample holder adapted for Falcon-type vials, ensuring laser safety during operation.
Spectral analysis was complemented with data obtained through high-performance liquid chromatography (HPLC), the reference method used to determine the actual ethanol concentration in the samples. These data were used to train and validate the Raman spectroscopy predictive models. The target ethanol concentration in the final product was set below 0.1% v/v, and the training samples covered a full range from 0% to 10% v/v ethanol.
Image of In-Line™ Raman analyzer system mounted on a mobile rack.
Development of the Predictive Model with Visum Master™
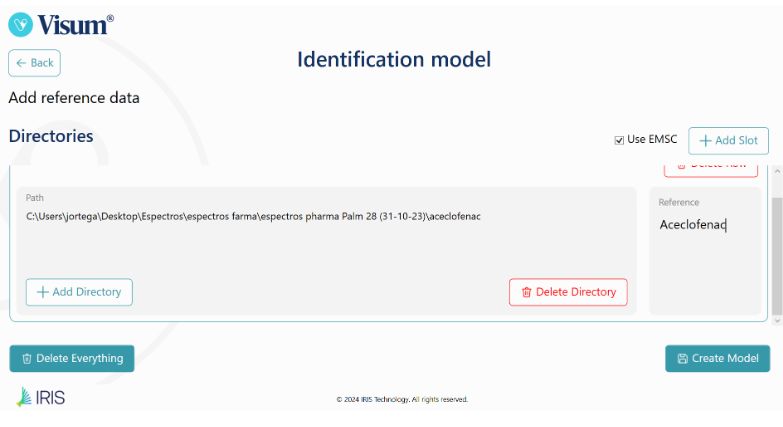
For developing the predictive model, synthetic samples were prepared in the laboratory by mixing water with varying ethanol concentrations, specifically designed to span the entire operational range expected in the real process. These samples ensured that the model could make accurate predictions across the entire interval of interest.
The Raman spectra obtained using the Visum Raman In-Line™ from these solutions were used to develop the predictive model with Visum Master™, an automated software platform that allows any user to create calibrations without needing advanced chemometric knowledge. The software automatically selected the most appropriate algorithm, optimal preprocessing methods (Savitzky-Golay derivatives, mean centering, baseline correction, etc.), and configured the final model using the calibration samples and their reference values (determined by HPLC).
The resulting models were later validated using an independent set of real samples collected during experimental liposome production.
Results and Evaluation of the Raman Predictive Model for Residual Ethanol
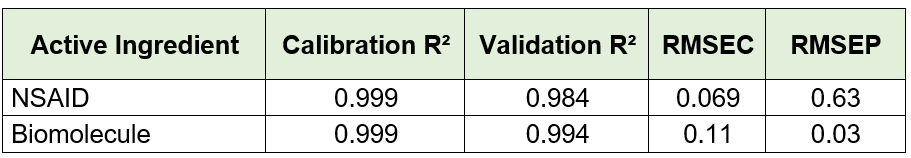
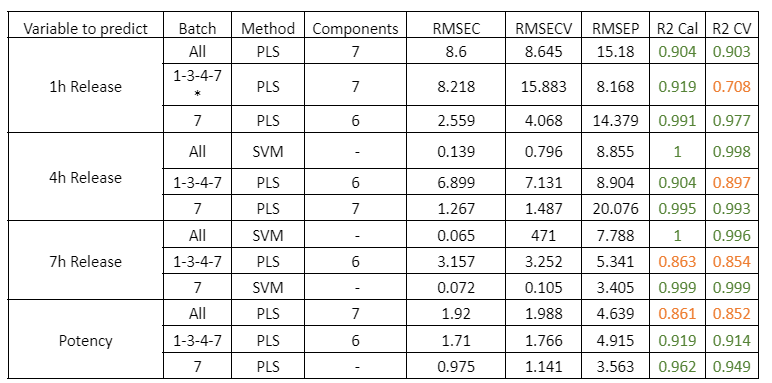
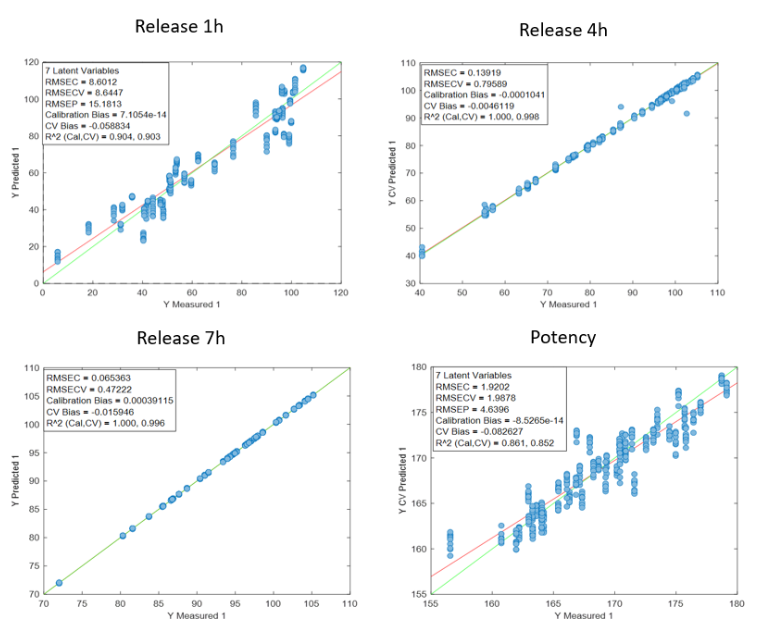
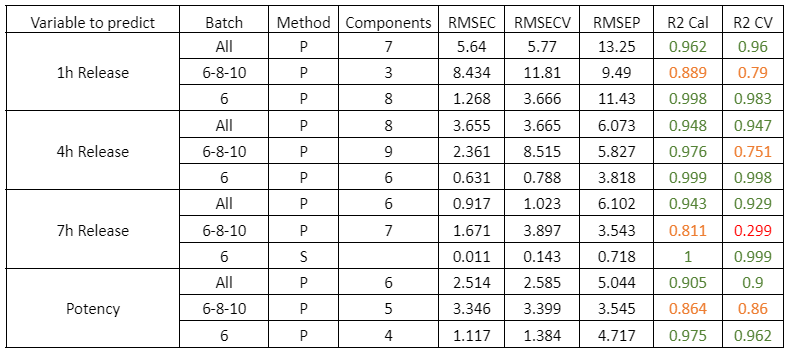
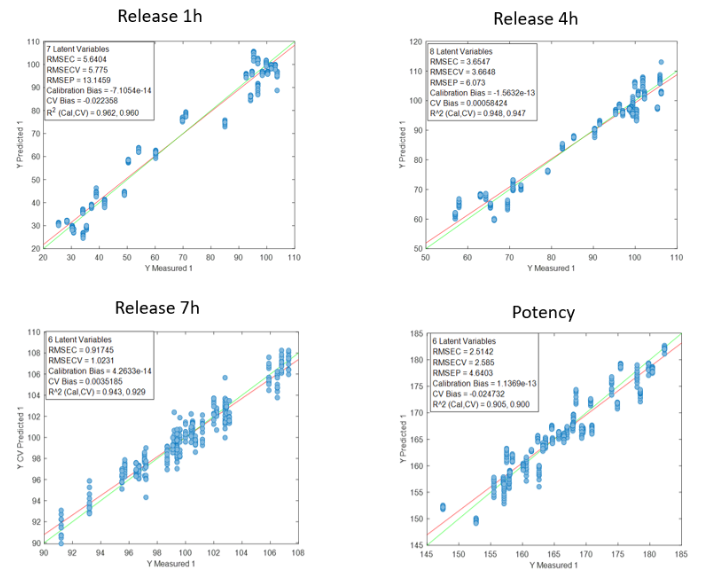
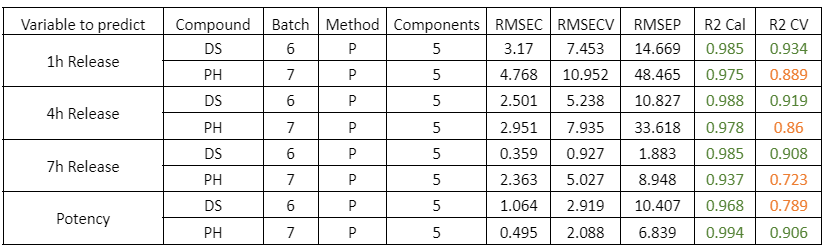
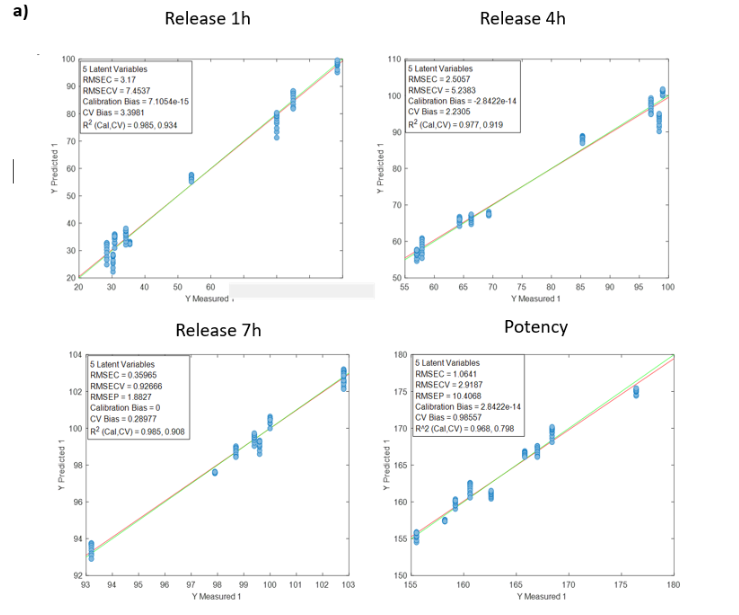
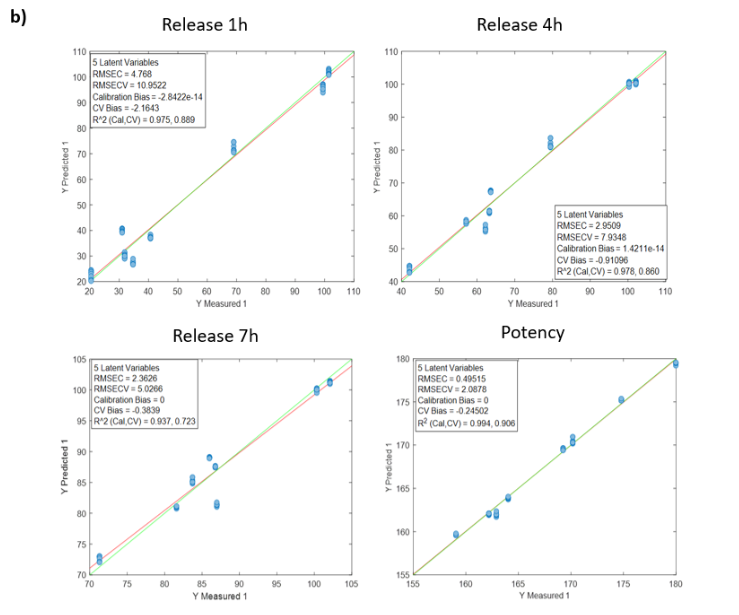
The models developed using Raman spectroscopy for quantifying active ingredients and residual compounds demonstrated excellent performance in both calibration and external validation. For ethanol, the model achieved a coefficient of determination (R²) above 0.99 for both calibration and validation, with a root mean square error of prediction (RMSEP) below 0.35% v/v, confirming its applicability for real-time monitoring during drying or cleaning processes.
The consistently low bias in all cases supports the robustness of the calibration and the absence of systematic errors, confirming the viability of Raman spectroscopy as a reliable technology for the quantitative monitoring of active ingredients in critical pharmaceutical processes.
Conclusions
The use of Raman spectroscopy through the industrial Visum Raman In-Line™ analyzer enabled the development of a robust and accurate model for ethanol monitoring during the diafiltration step in pharmaceutical processes. The high correlation and low deviation of results confirm the system’s suitability for real-world applications both in the laboratory—mounted on a mobile rack—and in in-line monitoring processes. Raman spectroscopy can also be extended to other pharmaceutical processes requiring precise control of solvents or critical compounds, enhancing operational efficiency and final product quality.